Mass Spectrometry (MS)- Principle, Working, Parts, Steps, Uses

Mass Spectrometry (MS) is an analytical chemistry technique that helps identify the amount and type of chemicals present in a sample by measuring the mass-to-charge ratio and abundance of gas-phase ions.
In this instrumental technique, the sample is converted to rapidly moving positive ions by electron bombardment and charged particles are separated according to their masses.
A mass spectrum is a plot of relative abundance against the ratio of mass/charge (m/e).
These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical structures of molecules and other chemical compounds.
Table of Contents
Interesting Science Videos
Principle of Mass Spectrometry (MS)
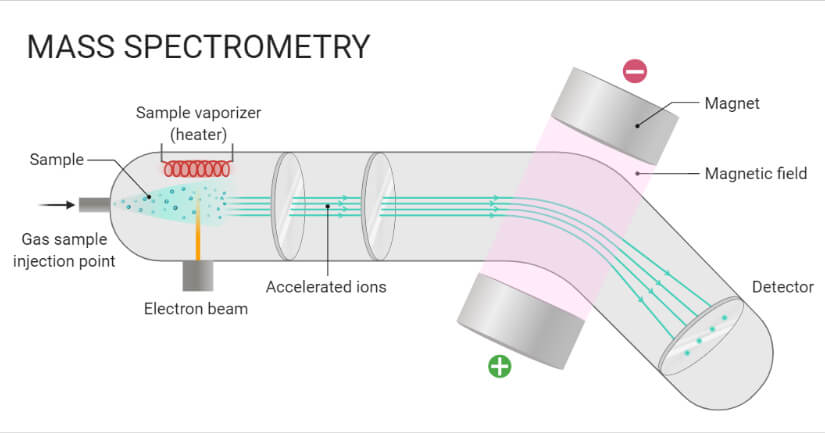
- In this technique, molecules are bombarded with a beam of energetic electrons.
- The molecules are ionized and broken up into many fragments, some of which are positive ions. Each kind of ion has a particular ratio of mass to charge, i.e. m/e ratio (value).
- For most ions, the charge is one, and thus, the m/e ratio is simply the molecular mass of the ion.
- The ions pass through magnetic and electric fields to reach the detector where they are detected and signals are recorded to give mass spectra.
Working of Mass Spectrometry (MS)
- In a typical procedure, a sample, which may be solid, liquid, or gas, is ionized, for example by bombarding it with electrons.
- This may cause some of the sample’s molecules to break into charged fragments. These ions are then separated according to their mass-to-charge ratio, typically by accelerating them and subjecting them to an electric or magnetic field:
- Ions of the same mass-to-charge ratio will undergo the same amount of deflection.
- The ions are detected by a mechanism capable of detecting charged particles, such as an electron multiplier. Results are displayed as spectra of the relative abundance of detected ions as a function of the mass-to-charge ratio.
- The atoms or molecules in the sample can be identified by correlating known masses (e.g. an entire molecule) to the identified masses or through a characteristic fragmentation pattern.
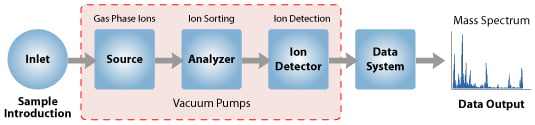 Instrumentation and Steps of Mass Spectrometry (MS)" width="535" height="125" />
Instrumentation and Steps of Mass Spectrometry (MS)" width="535" height="125" />
A. Sample Inlet
- A sample stored in the large reservoir from which molecules reach the ionization chamber at low pressure in a steady stream by a pinhole called “Molecular leak”.
B. Ionization
- Atoms are ionized by knocking one or more electrons off to give positive ions by bombardment with a stream of electrons. Most of the positive ions formed will carry a charge of +1.
- Ionization can be achieved by :
- Electron Ionization (EI-MS)
- Chemical Ionization (CI-MS)
- Desorption Technique (FAB)
C. Acceleration
- Ions are accelerated so that they all have the same kinetic energy.
- Positive ions pass through 3 slits with voltage in decreasing order.
- Middle slit carries intermediate and finals at zero volts.
D. Deflection
- Ions are deflected by a magnetic field due to differences in their masses.
- The lighter the mass, the more they are deflected.
- It also depends upon the no. of +ve charge an ion is carrying; the more +ve charge, the more it will be deflected.
E. Detection
- The beam of ions passing through the mass analyzer is detected by a detector on the basis of the m/e ratio.
- When an ion hits the metal box, the charge is neutralized by an electron jumping from the metal onto the ion.
- Types of analyzers:
- Magnetic sector mass analyzers
- Double focussing analyzers
- Quadrupole mass analysers
- Time of Flight analyzers (TOF)
- Ion trap analyzer
- Ion cyclotron analyser
Applications of Mass Spectrometry (MS)
- Environmental monitoring and analysis (soil, water, and air pollutants, water quality, etc.)
- Geochemistry – age determination, soil, and rock composition, oil and gas surveying
- Chemical and Petrochemical industry – Quality control
- Identify structures of biomolecules, such as carbohydrates, nucleic acids
- Sequence biopolymers such as proteins and oligosaccharides
- Determination of the molecular mass of peptides, proteins, and oligonucleotides.
- Monitoring gases in patients’ breath during surgery.
- Identification of drug abuse and metabolites of drugs of abuse in blood, urine, and saliva.
- Analyses of aerosol particles.
- Determination of pesticides residues in food.
References and Sources
- https://www.thermofisher.com/au/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-mass-spectrometry.html
- https://www.slideshare.net/akshukumarsharma/mass-spectroscopy 55382941
- http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch13/ch13-ms.html
- https://en.wikipedia.org/wiki/Mass_spectrometry
- https://www.chemguide.co.uk/analysis/masspec/howitworks.html
- https://www.slideshare.net/solairajananant/mass-spectrometry-38534267
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/massspec/masspec1.htm
About Author
Sagar Aryal is a microbiologist and a scientific blogger. He is doing his Ph.D. at the Central Department of Microbiology, Tribhuvan University, Kathmandu, Nepal. He was awarded the DAAD Research Grant to conduct part of his Ph.D. research work for two years (2019-2021) at Helmholtz-Institute for Pharmaceutical Research Saarland (HIPS), Saarbrucken, Germany. Sagar is interested in research on actinobacteria, myxobacteria, and natural products. He is the Research Head of the Department of Natural Products, Kathmandu Research Institute for Biological Sciences (KRIBS), Lalitpur, Nepal. Sagar has more than ten years of experience in blogging, content writing, and SEO. Sagar was awarded the SfAM Communications Award 2015: Professional Communicator Category from the Society for Applied Microbiology (Now: Applied Microbiology International), Cambridge, United Kingdom (UK). Sagar is also the ASM Young Ambassador to Nepal for the American Society for Microbiology since 2023 onwards.




 Instrumentation and Steps of Mass Spectrometry (MS)" width="535" height="125" />
Instrumentation and Steps of Mass Spectrometry (MS)" width="535" height="125" />